Editor’s Note: For exclusive access to download this complete deck, please sign up for our a16z Bio Newsletter here.
The new wave of technologies being used to rewrite our genetic code is becoming one of the most powerful therapeutic arsenals of all time. This next paradigm of medicine brings with it a whole new set of opportunities and challenges. In this deck, I walk through the scientific breakthroughs driving innovations in gene therapies, as well as the biggest hurdles to solve in order to reach many more patients.
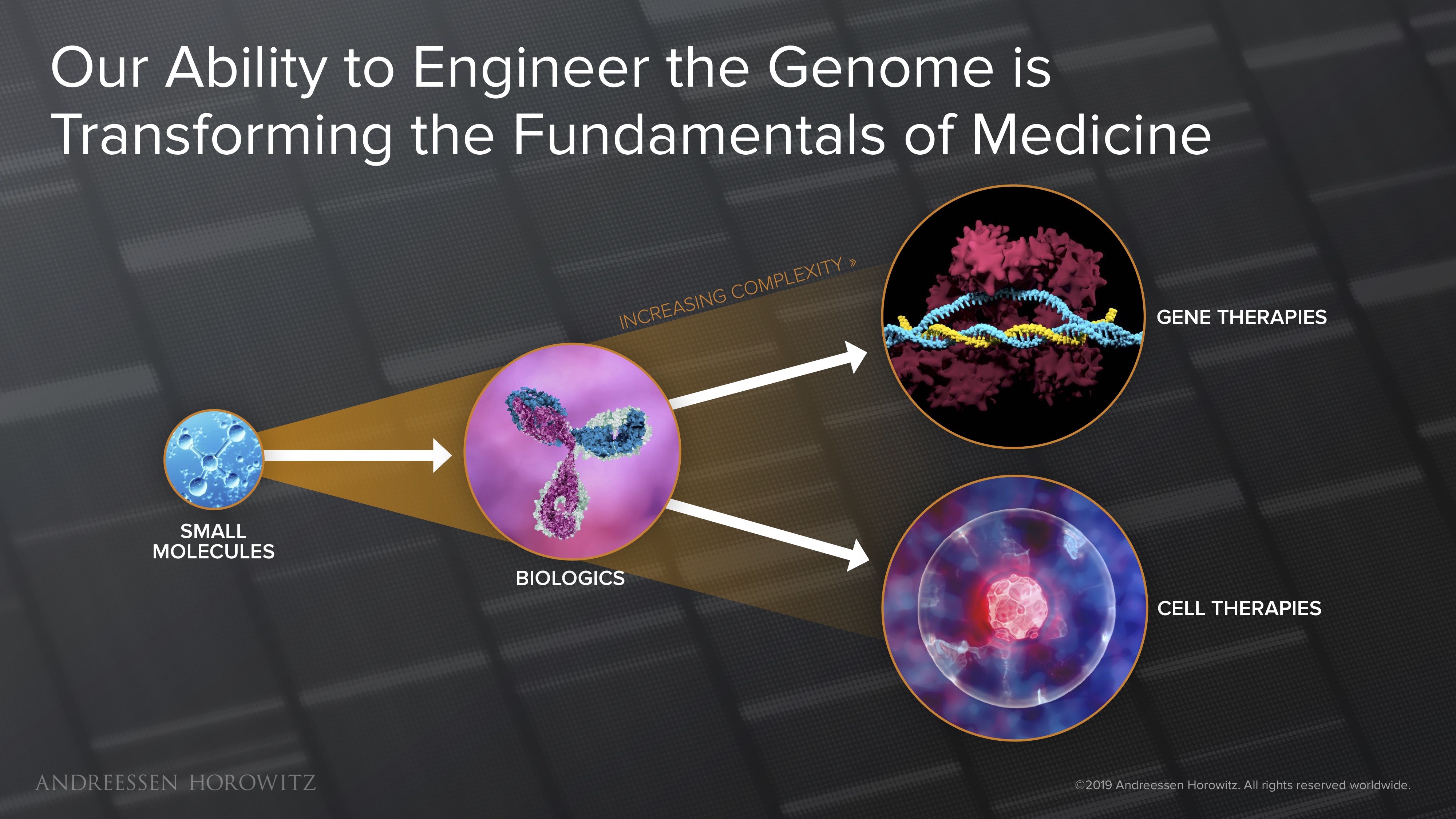
Our medicines are now more powerful and complex than ever before. We’ve gone from small molecules, to full antibodies, to using biological machinery to engineer our genomes to cure disease. These technologies have given us the power to engineer personalized treatments that: a) precisely target and eliminate the origin of disease; and b) can potentially be curative, with a single dose of drug.
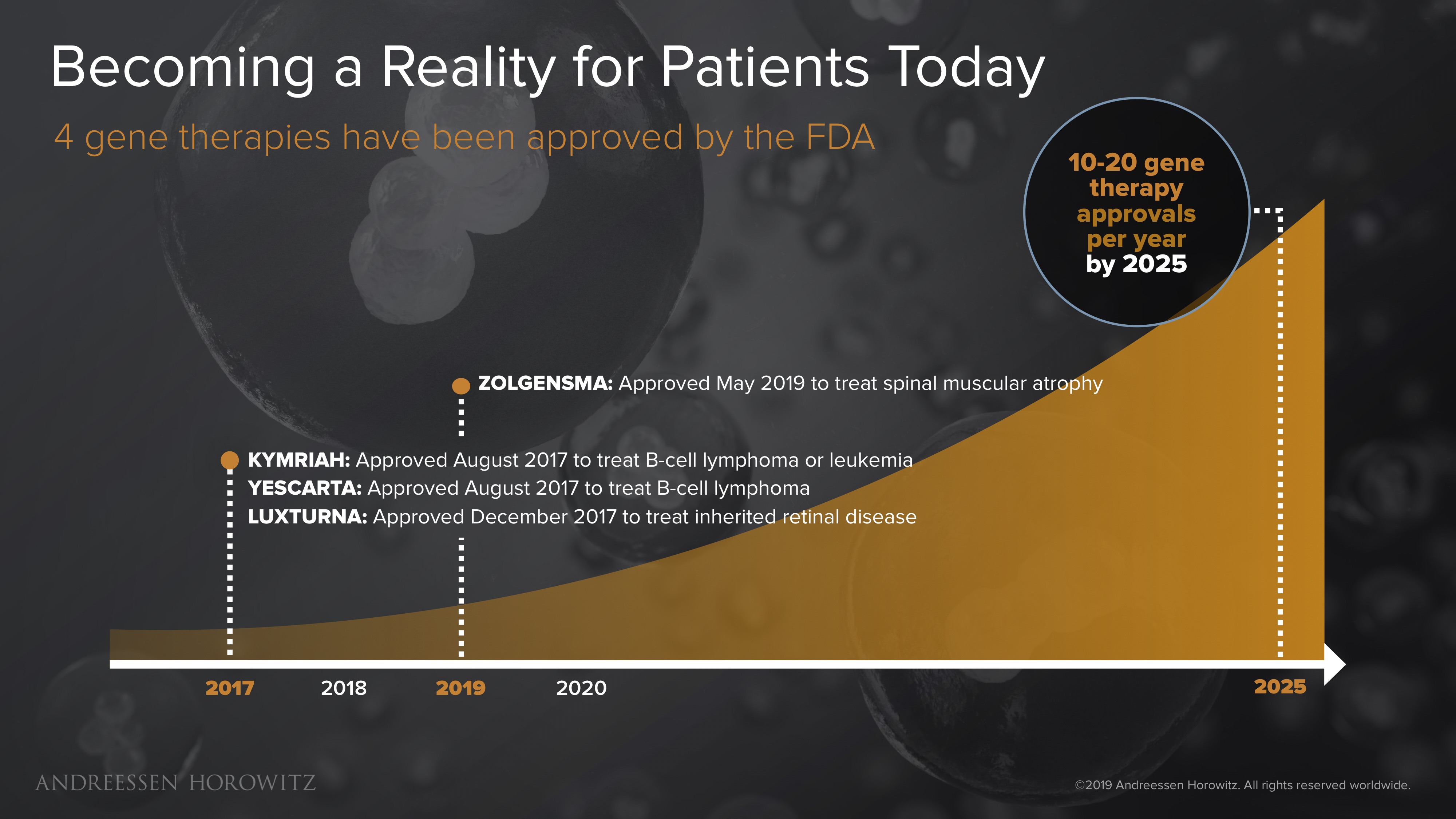
We are really just at the beginning of this frontier. The FDA recently approved 4 gene therapy treatments for patients—a pivotal milestone in the history of medicine. Excitingly, the most recent 2 approvals (Luxturna for a form of congenital blindness, and Zolgensma for spinal muscular atrophy) have been actual in-vivo gene therapies—meaning these complex medicines are delivered to combat the genetic mutation directly inside the body.
The promise of these breakthroughs has created enormous opportunity for startups working on these genetic medicines. Some of the largest biotech exits in recent years have been from companies developing gene therapies. As the pace of innovation continues to accelerate, and the clinical landscape and regulatory environments continue to get familiar with this new modality, the opportunity size will only continue to grow. The industry expects to see at least 10-20 gene therapy approvals per year, by the end of the next decade. That is an order of magnitude more ‘one-and-done’ cures than we have ever seen before.
How We Got Here
To better appreciate this huge technology and paradigm shift, we need to understand how we got here. One of the biggest enablers of this massive new wave of innovation is a whole new level of precision and predictability. The arc of this field has gone from a largely stochastic, random genetic mechanism, to something that is, in essence, engineering. Below, I walk through the key approaches we’ve developed over time, and where we are now.
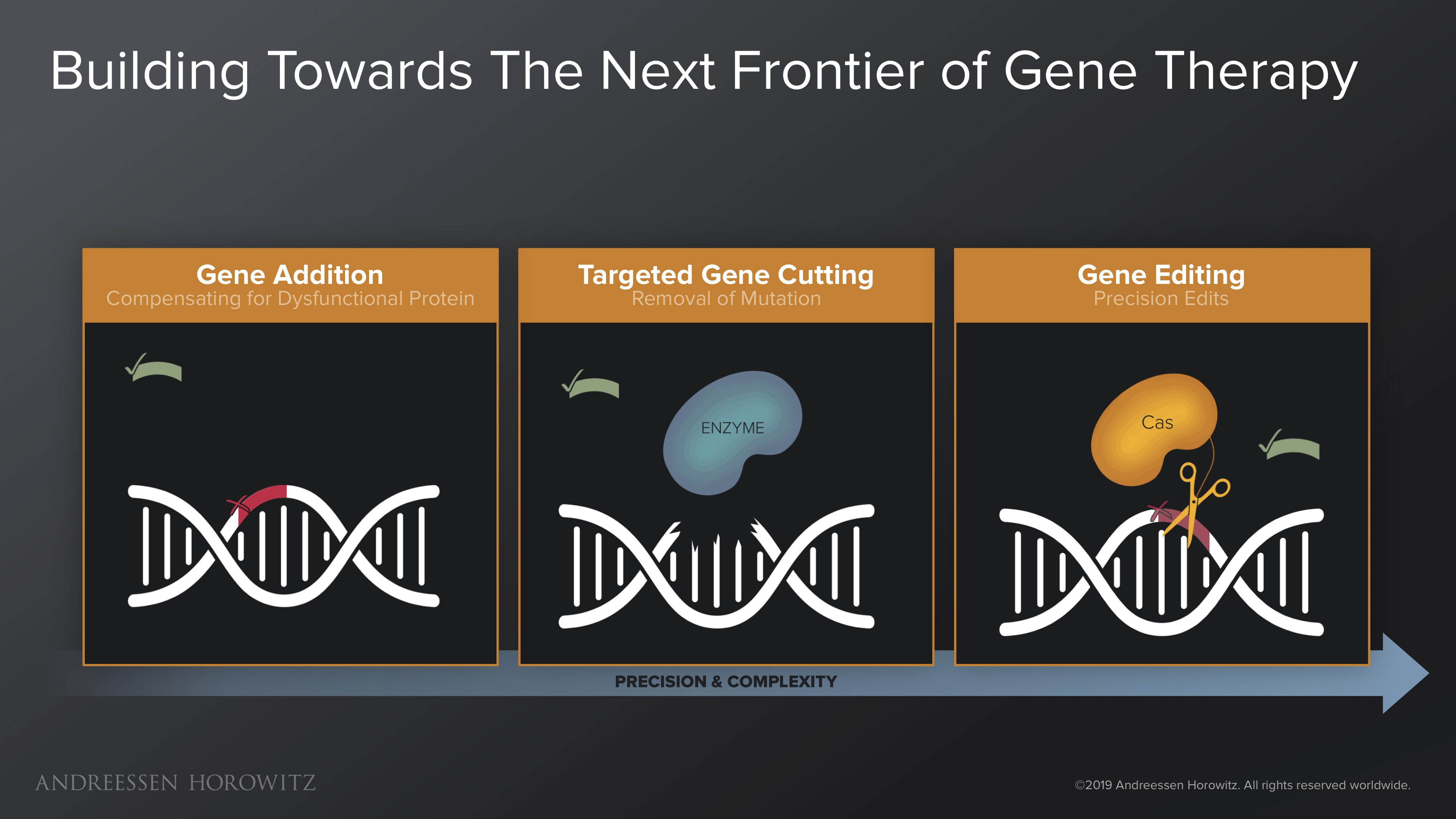
Gene Addition: Early approaches to gene therapy were pretty rudimentary. At first, gene therapy was just simple transgene addition—which means we essentially jammed healthy copies of a gene into a diseased cell. The hope was that these healthy copies would produce enough functional proteins to compensate for the mutated gene. The problem with this method is that it never really removes the root cause mutation. Some tragic learnings from harsh early clinical trial realities brought scientists and physicians to agreement that jamming in foreign genes at random places without fixing the root cause is not a viable strategy for therapy.
Targeted Gene Cutting: We then progressed to focus on making proteins that can target and cut the source of the mutated gene, in the form of double-stranded breaks—thereby inactivating the root cause mutation. The first tools created for this (meganucleases, zinc fingers and TALENs) worked, but were clunky, expensive, and laborious to make; you had to design individual proteins to bind to a certain genetic sequence. So they were never widely adopted for experimentation and practical use. But the approach did lay the foundation and proof of concept for a more precise paradigm of genetic manipulation.
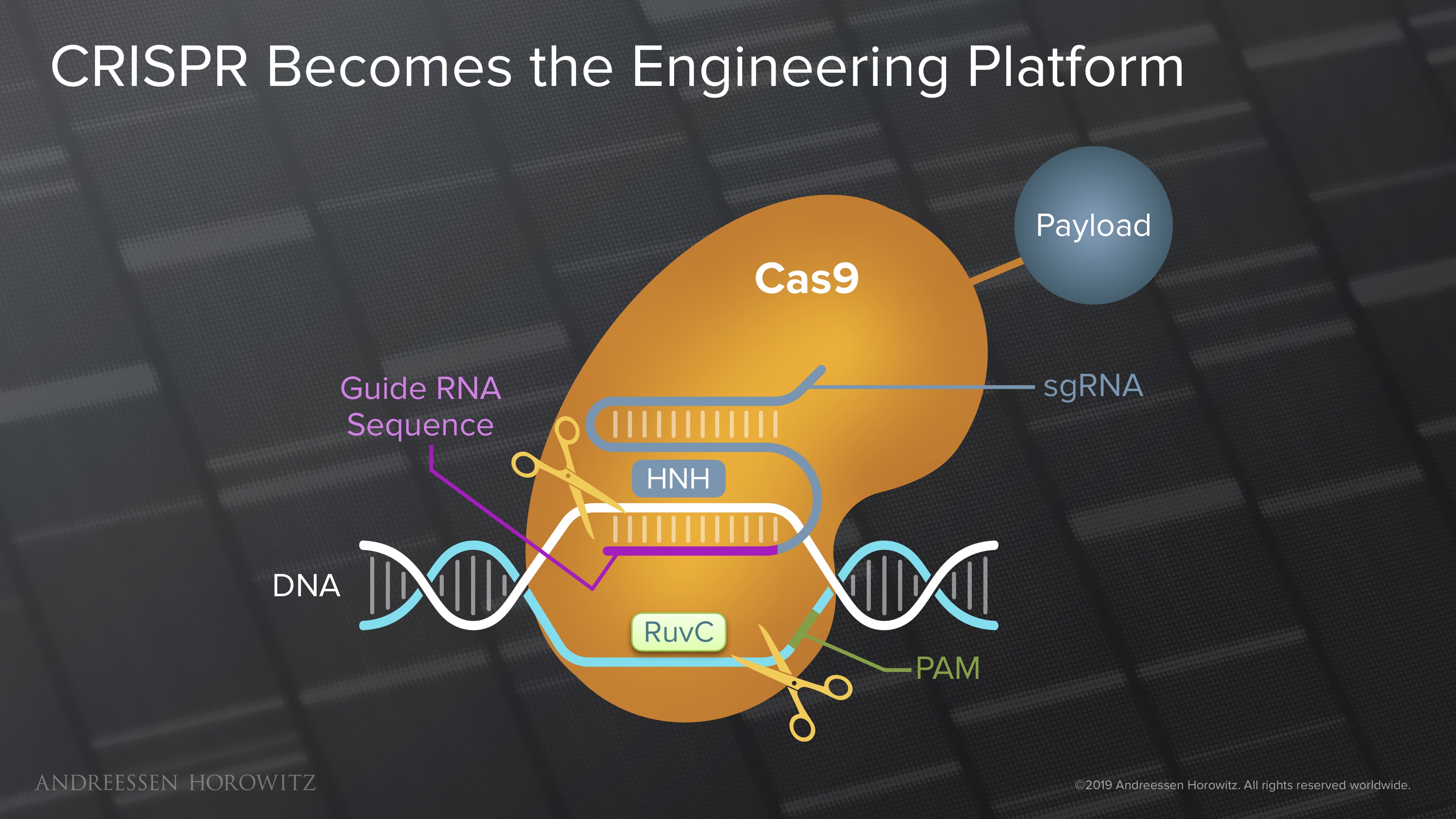
Gene Editing: The field really got its lucky break from nature in the discovery of the CRISPR Cas9 nuclease (from Streptococcus bacteria). This was a huge leap forward, in which programming a simple RNA strand can enable targeting of most genomic locations—a very elegant and efficient system for genome editing. Manipulation at the genome scale was cut down from months, to weeks or even days. The early wave of CRISPR Cas9 gene editing was not perfect, and its double-stranded break mechanism was muddled by off-targets effects. But as a tool, it was a key building block to a scalable and programmable future for genome engineering. R&D became much more iterable; the scientific community adopted this like wildfire.
Precision Genome-wide Engineering
The latest generation of CRISPR tools coming online are truly pushing us into the realm of precision genome engineering. Not only have these nucleases been better optimized and refined by the massive scientific and engineering community, the genome “developer ecosystem” is now building totally new functionality on top of this platform. Clean, precision editing is just the start.
CRISPR is no longer just the Cas9 nuclease. We can now look to an entire ecosystem of proteins that build out the genome engineering toolbox. Some nucleases are much smaller, some come from less immunogenic strains, others are more precise, or have new biological mechanisms altogether.
All of these characteristics and properties are enabling applications that were previously unimaginable. As CRISPR Cas9 was a serendipitous discovery from studying Streptococcus bacteria, scientists have been on the hunt sifting through samples from unexplored ecosystems (hydrothermal vents, geysers or even city subway stations) to mine for other novel proteins from microbial species. There is even work to develop molecular systems that recruit the cell’s own natural DNA modifying proteins (endogenous cellular machinery) to do genomic editing. We are literally only scratching the surface of new molecular technologies.
The biggest recent shift in the field is the push towards making CRISPR a “search engine platform of the cell.” In other words, CRISPR has become the molecular infrastructure in which new genomic targeting technologies are built. Many powerful new applications in genome engineering take the CRISPR Cas proteins as a blueprint, and replace it with other protein domains that can manipulate the genome in new ways. Once the catalytic cutting domains responsible for double-stranded breaks are inactivated, we can attach new payloads or new molecular tools to manipulate the genome.
Quick and targeted gene knockouts was clearly just the start. It is this new CRISPR 2.0 era that allows us to manipulate the genome in a huge new range of dimensions that were fundamentally not possible before. We can now replace the catalytic cutting domains with CRISPR activators and repressors to push genes into overdrive, or repress them directly at the source, for example. Scientists have even developed deaminases that can edit the genome at a single base resolution. We can even directly target and edit RNA bases, opening up the entire field of transcriptomic editing. This level of precision opens the door to addressing an entire field of devastating diseases that were previously untreatable before.
Every few months—or even weeks—we see powerful new tools that are built using this system that allows us to unlock new engineering possibilities. But direct applications to new wave of therapeutics is just the start. We are seeing diagnostics or even molecular imaging that use CRISPR as the platform layer for applications that require precise genomic targeting. New uses cases are just waiting to be developed with this easily programmable toolkit.
Building a Gene Therapy Company
In sum, we think there is no better time to build the next generation gene therapy company. There are a couple of factors at play here:
- Science becomes Engineering: One of the most exciting things to see is when cutting edge scientific advances transition into an engineering platform that can be democratized and broadly built upon (e.g., the latest wave of CRISPR innovation).
- Massive Developer Community Flywheel: Because of the simplicity and ease of iteration of this engineering platform, a powerful and very active developer community has sprung up—one that is continually refining and building new superpowers to catalyze the entire field.
- Established Market: As these technologies are translated into medicines that are approved, commercialized, and administered to patients, they in turn establish a robust clinical and commercial precedent for new entrants to the space.
- Favorable Regulatory Environment: This past year we’ve seen FDA and CBER (Center for Biologics Evaluation and Research) leaders lay plans to make a big push on assessing new cell and gene therapies, as well as introduce new guidance on accelerated approvals for these new areas of product development.
All these factors driving towards a market that is constantly growing (with even more patients waiting) puts us squarely in this perfect moment for gene therapy innovation.
Still to be solved…
However exciting the prospects of these powerful new drugs may seem, it does come at the expense of the layers of added complexity. Not only must we continue to refine the science and engineering, we need to develop the infrastructure and value chain to support, manufacture and ultimately deliver these tailored cures to the patient.
Some of the core challenges that need to be solved:
- Delivery Issues: Getting these complex medicines into the target organ and ultimately specific cells that you want is no trivial feat.
- Immune response: The drug should trigger minimal immune responses that neither neutralize the effect of the drug or provoke lethal side effects.
- Molecular Precision: Precision of the genomic targeting—to prevent any unintentional collateral damage to the rest of your healthy genome—is critical.
- Editing Efficiency: Efficiency and ultimately potency of the drug must be carefully optimized (without overloading the body with unsafe concentrations of the drug).
- Manufacturing: How do we scale up the production of these complex, bespoke cures for hundreds of thousands—and eventually millions—of patients?
Companies in the space are focusing on solving these issues in a few key ways:
- Delivery System Engineering (Can we create better delivery systems?)
- Toolbox & Protein Engineering (Can we better optimize or engineer the gene editing machinery itself, developing new modalities and expand the genome engineering toolbox?)
- AI and Data Mining (Can new AI innovations help us refine our R&D, make our drugs safer, uncover new systems, or even allow us to harness new biological mechanisms?)
- Manufacturing Infrastructure (Can we apply the learnings from software, connected hardware, and automation from the tech industry to rejuvenate a dated manufacturing infrastructure?)
The beauty of these key areas is that because they are so core to the industry and important for every drug, that each solution has the potential to develop massive scale and value for the broader gene therapy ecosystem.
I’ll walk through each category below in some more detail to zoom into some of the innovations in the space.
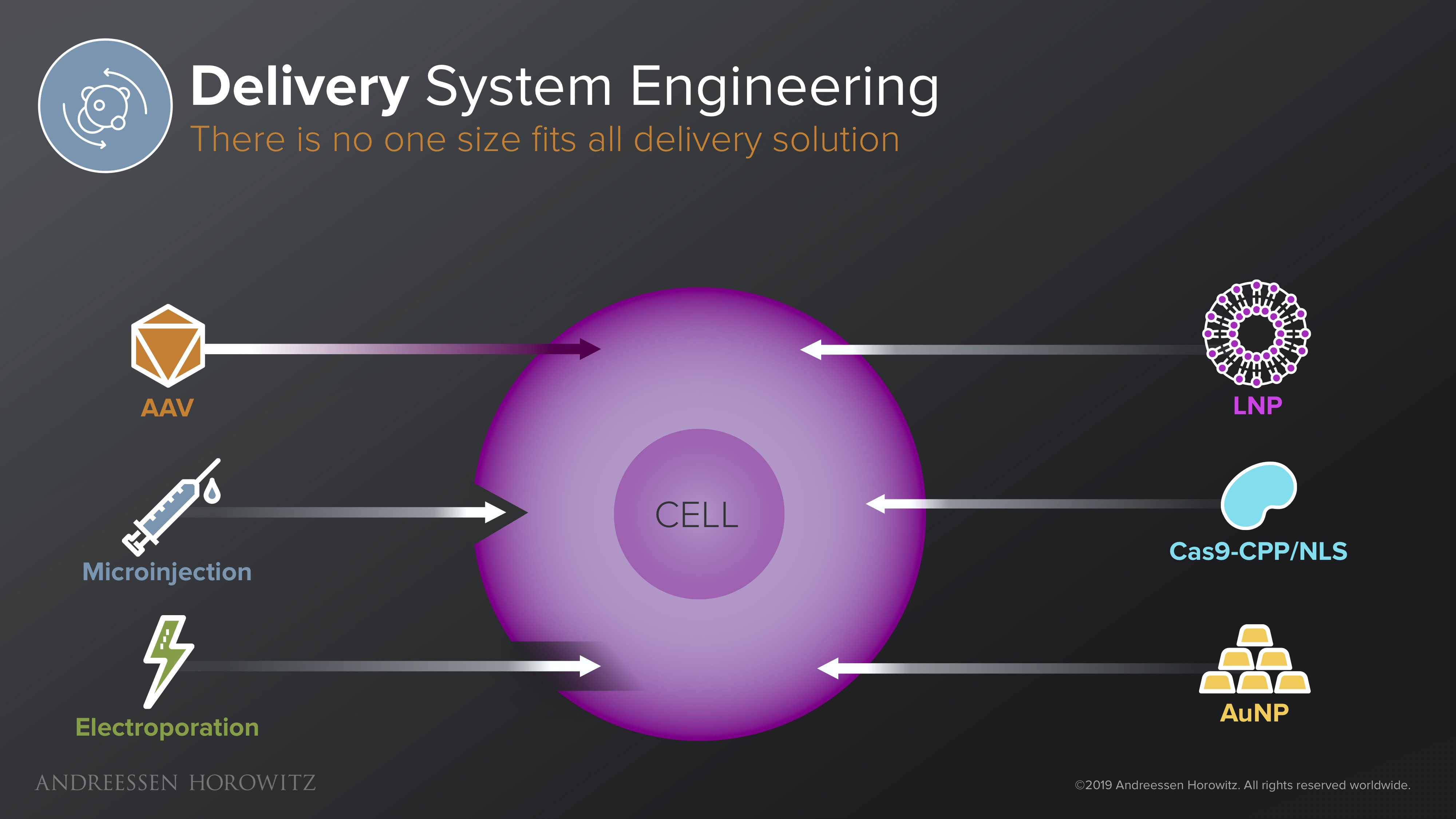
Delivery Solutions
Delivery is one of the central components of every single gene therapy. It doesn’t matter how great the therapy is if it never gets inside the cell, or if they are provoke a lethal immune response. Many delivery vectors are currently plagued with low organ-level precision, low efficiency, and/or high immunogenicity.
Below are some of the solutions in different delivery mechanisms categories we are beginning to see:
Viral Vectors: AAVs (adeno-associated viruses) are the only clinically approved vector for gene therapy so far. But it’s hardly a no-brainer solution. Since, immunogenicity is a huge challenge with AAVs, most of these therapies are limited to few immune-privileged organs. They are also a non-starter for any patients with pre-existing immunity, and cannot be re-dosed because of the formation of neutralizing antibodies. Some startups are working on novel platforms for capsid discovery and engineering from developing new high-throughput directed evolution techniques to leveraging in-silico ML-based approaches to develop vectors with lower immunogenicity or programmable tropism. There are also other efforts engineering clever new biological systems made to mask viral vectors from the immune system or exploring different species altogether. The main goal here is to take a validated, but rather narrow system and engineer it for the much broader next generation of gene therapies.
Non-Viral Vectors: In the category of non-viral vectors, there is a wide array of materials as potential solutions, from lipid nanoparticles, various chemical polymers, exosomes, to other systems that have some biomimetic functionality. In addition to being redosable and solving the packaging constraints of AAVs, some of these solutions are also less complex or costly to manufacture. For many applications—diseases targeting the liver, for instance—this might be enough. For other diseases, in vivo targeting of certain organ systems might be tougher than using viruses, and unpacking kinetics might be slow, so there is also lots of optimization and targeting moieties that can potentially be engineered.
Ex-vivo Delivery: For ex-vivo gene therapies that can take advantage of delivery outside of the body, startups have been working on technologies and hardware that can disrupt the cell membrane en masse, and deliver larger cargo, on a quicker time-scale. Some of these membrane disruption technologies (like electroporation, or other fluidic based disruption techniques) allow for rapid delivery of a diverse range of materials, in diverse cell types, without the use of any vectors. The challenge here is to apply technologies that inherently utilizes harsh physicals forces while minimizing damage to the membrane and proteins. For specific hardware approaches (such as microinjections, or various microfluidic devices), the question becomes how to scale it up.
There are a wide variety of potential solutions and no silver bullet here. Regardless of the type of vector you are engineering, we believe the winners in this space are the ones that can harness a specific platform and marry it with very thoughtful optimization towards specific disease areas.
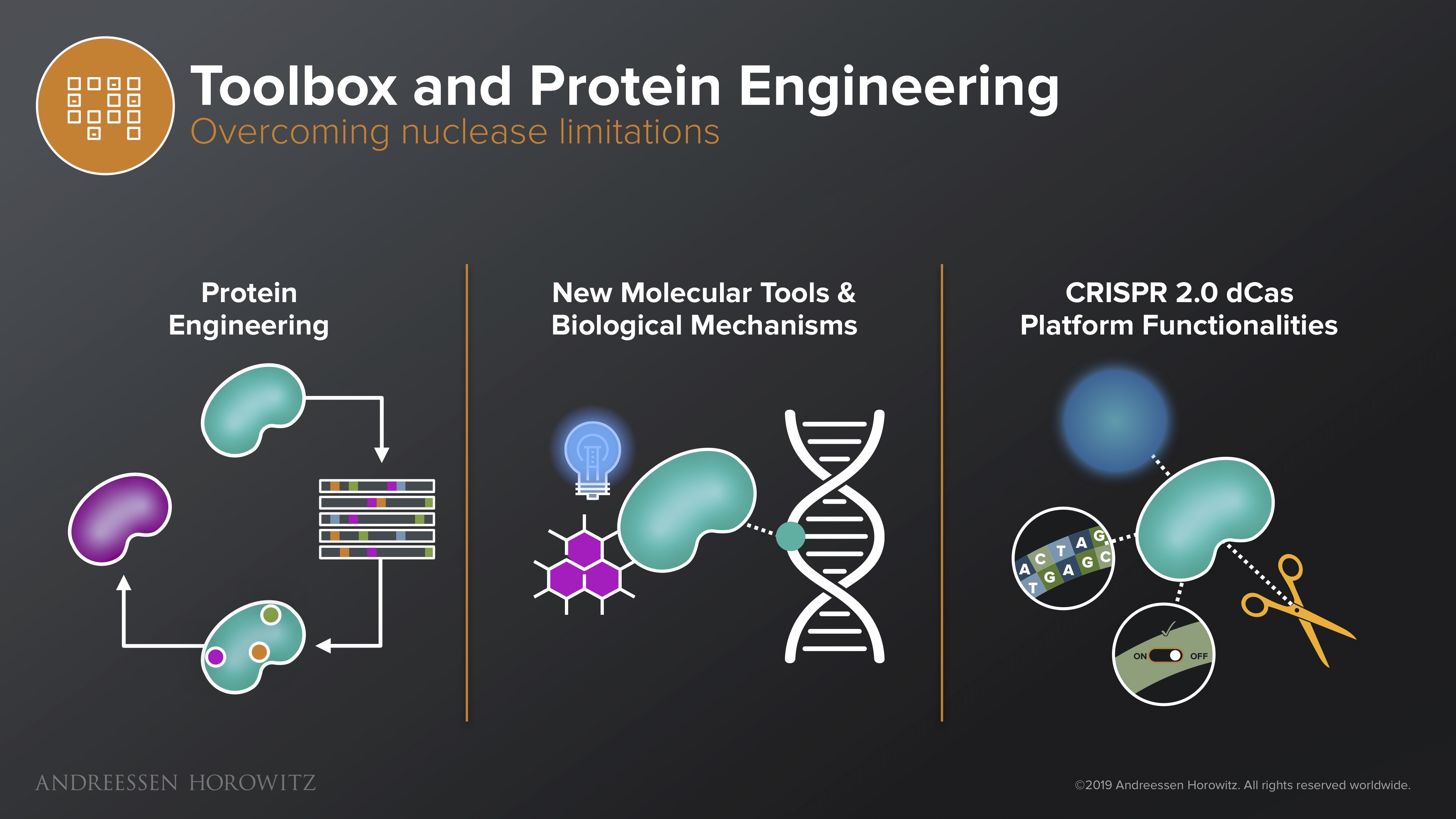
Toolbox and Protein Engineering
Though delivery is immensely important, it is just getting to where you need to go. Once inside the cell, many of our current protein-based genome editing nucleases are still plagued by off-target effects, low efficiency, and other side effects from immunogenicity of the protein itself (not too surprising, since our most popular CRISPR Cas9 protein comes from staph and strep bacteria).
Below are some approaches currently being developed to deal with these issues:
Protein Engineering: Since the central core of the technology is based around the nuclease itself, a lot of the solutions can come from the protein engineering angle. Startups here are developing clever systems to do scalable, high-throughput protein engineering. These systems attack CRISPR’s inherent technical challenges by engineering nucleases that may have expanded PAM, higher specificity binding nucleases, and optimizing other variants that may have unique properties such as smaller size, higher efficiency and lower immunogenicity.
New Molecular Tools and Biological Mechanisms: There are also a lot of creative efforts that marry advances in synthetic biology and chemistry to develop new tools that better manipulate or regulate our gene-editing enzymes. For instance, scientists have programmed new on-off switches for the Cas enzyme to add levels of control or engineered photocleavable domains so that CRISPR is only activated when activated by light, allowing for spatial and temporal manipulation of the system.
CRISPR 2.0 Platform Functionalities: Even more exciting, some are avoiding the conventional CRISPR platform altogether and using it as a foundational platform to build new tools that allow us to reprogram biology in new dimensions. Base editing, prime editing, transcriptomic editing, epigenetic modifications, etc, can not only sidestep the initial technical issues standard CRISPR double-stranded break mechanism is plagued by, but also allow us to manipulate our genome in a level of resolution that was never before possible.
There are also efforts that abandon the CRISPR nuclease altogether, utilizing technology that can recruit the cell’s endogenous machinery for genetic repair or RNA editing. Companies developing these systems improve the fundamental gene therapy toolkit, and unlock our ability to make drugs for a number of diseases that were untreatable before (or do so in a much safer way). All these innovations expand the power, breadth, and reach of our new therapeutic arsenal.
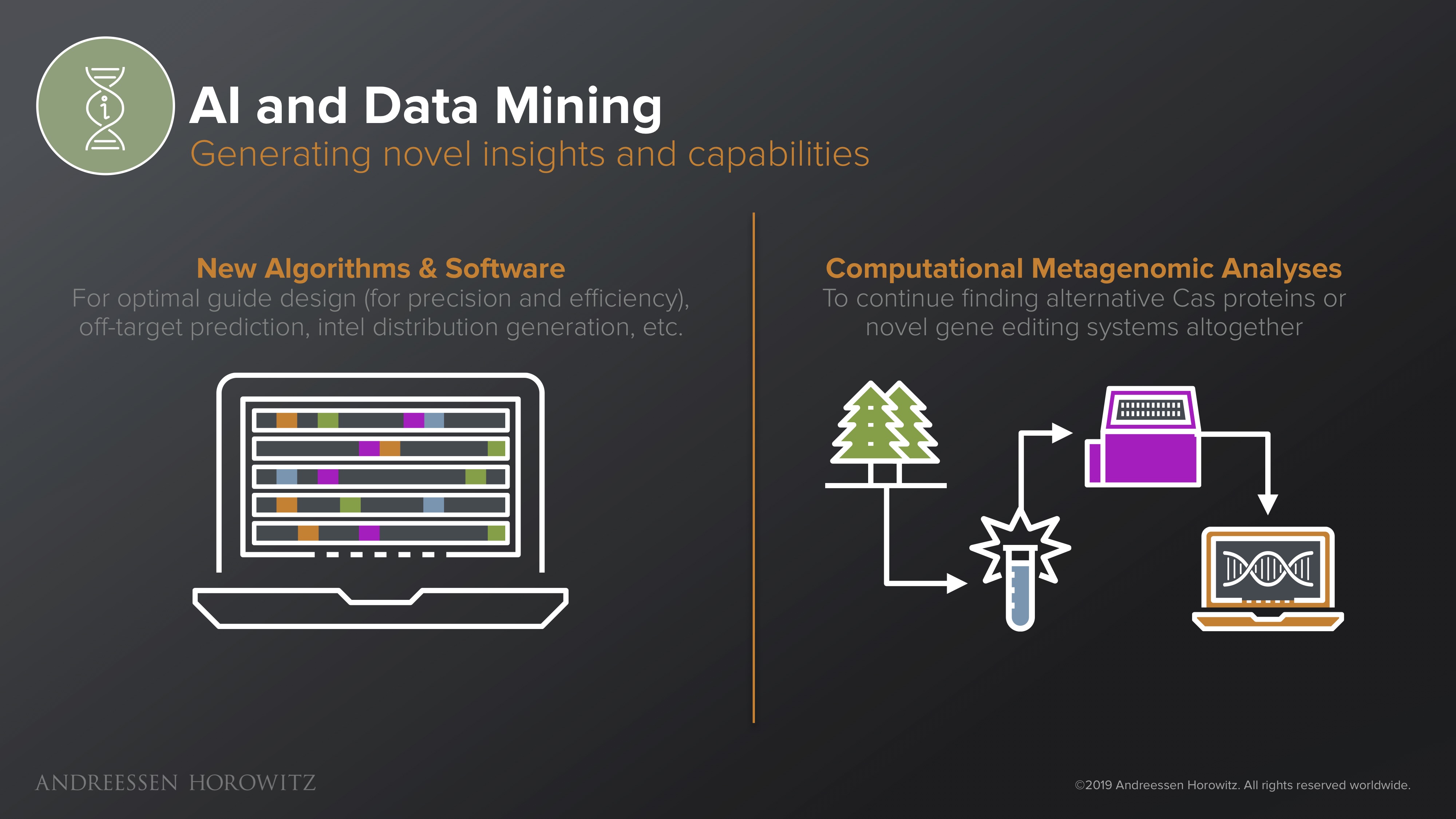
AI and Data Mining
There are many avenues on the computational front that help us with new insights. These insights can in turn create new super powers from old tools, or help us create new tools all together.
New Algorithms & Software: On the technical improvement side, software tools such as ML and data science help us to design gRNAs with higher efficiency and precision. ML-driven solutions that learn from prior assays help scientists make constructs that are much more adept at engineering difficult to edit cell types. On the analysis side, there are groups working on algorithms can predict beforehand where and how CRISPR will actually interact in a genome—essentially, a heatmap of how the system is likely to act or succeed vs fail. With these new predictive capabilities, scientists can engineer new therapies that are either more precise and safer, or even take advantage of these inherent flaws (if the system is deemed to yield the mutation you want).
Computational Metagenomic Analyses: Some efforts here are directed towards developing high throughput screening and metagenomic analysis engines that operationalize the process of mining for related nucleases or discover new genome editing systems. As we continue to analyze metagenomic data of new species, it’s not crazy to think that perhaps CRISPR is only the first frontier of genome engineering. The hope is that these new systems can have properties that ameliorate prior flaws, or have new superpowers all-together—smaller enzymes, non-immunogenic species, or novel biological mechanisms.
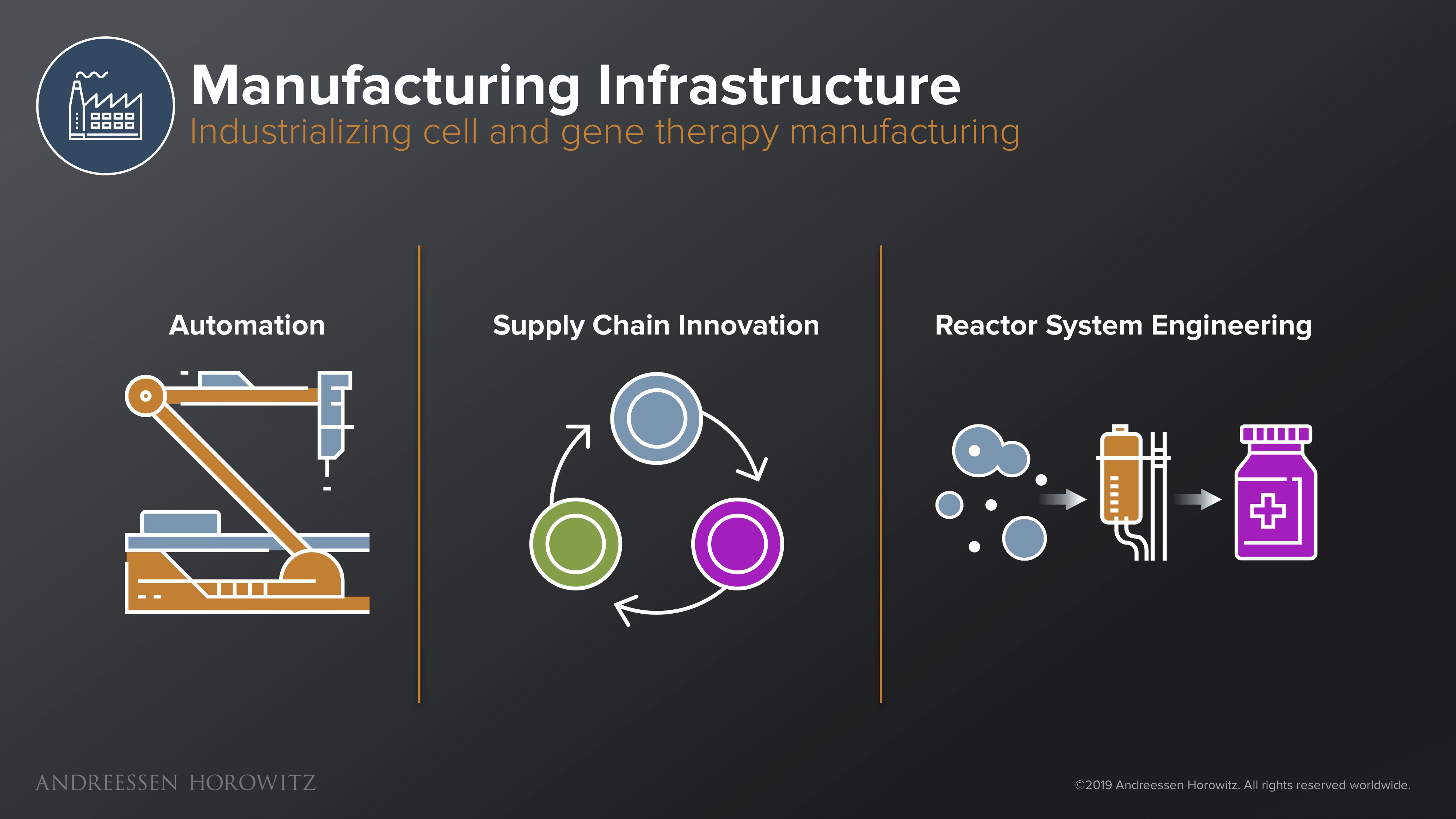
Manufacturing Infrastructure
Once you have designed the next potential gene therapy breakthrough, one of the most existential threats to this entire industry is manufacturing bottlenecks. There is a large, hairy system and a deep supply chain that has to work laboriously behind the scenes to make and deliver these complex drugs to patients. Innovation in this area is extremely important, because the current infrastructure is horrendously outdated and unprepared for the new influx of gene therapies in the clinical pipeline.
Below I describe a few key areas where solutions are needed/being developed.
Automation: One area of innovation can come from automation. This could be either new robotics that afford you newfound speed and scale, or modular systems that let you continue to experiment and adapt, using new vectors and different cell types without revamping an entire process. Automation can also come from the software side, in LIMS (Laboratory Information Management Systems) and other new ways to operationalize and scale these complex processes. Automated track and trace and data logging are also essential for safety. Having good software allows manufacturers and scientists to have clearer oversight on critical attributes associated with the product, and better control for the variability of the starting material.
Supply Chain Innovation: On the supply chain side, we need better software for real time tracking of cells and solutions that give us clearer visibility into the patient therapy’s development cycle. For instance, enterprise grade software that provides chain of identity or chain of custody at scale will be essential for clinical trust and safety. Connecting supply chain and courier systems, transport condition monitoring, and electronic batch record systems is all key.
Reactor System Engineering: Biology itself can even play a part in alleviating bottlenecks. For example, new innovations in synthetic biology can make better ‘producer’ cell lines that are able to produce viral vectors at much higher yields. Other advances in metabolic engineering or fermentation workflows and innovative bioreactors can also aid in producing plasmids that are better quality and higher yield.
What does all this mean for entrepreneurs?
There are many exciting angles to tackle the pressing challenges facing the gene therapy industry and massive opportunity to build new companies. Even things that may not have seemed like big businesses before can become immensely valuable because of the nature of the rapidly growing gene therapy ecosystem.
Those seemingly niche solutions for complex therapies are becoming a standard part of a new therapeutic value chain. Viral vector and cell manufacturing have become important core competencies of a variety of contract development and manufacturing organization and biotech service providers. And of course there are the companies that are taking the all the areas that we’ve just discussed, and building full-stack gene therapy companies that integrate everything from R&D to delivery system engineering to in-house manufacturing to development of complex therapies.
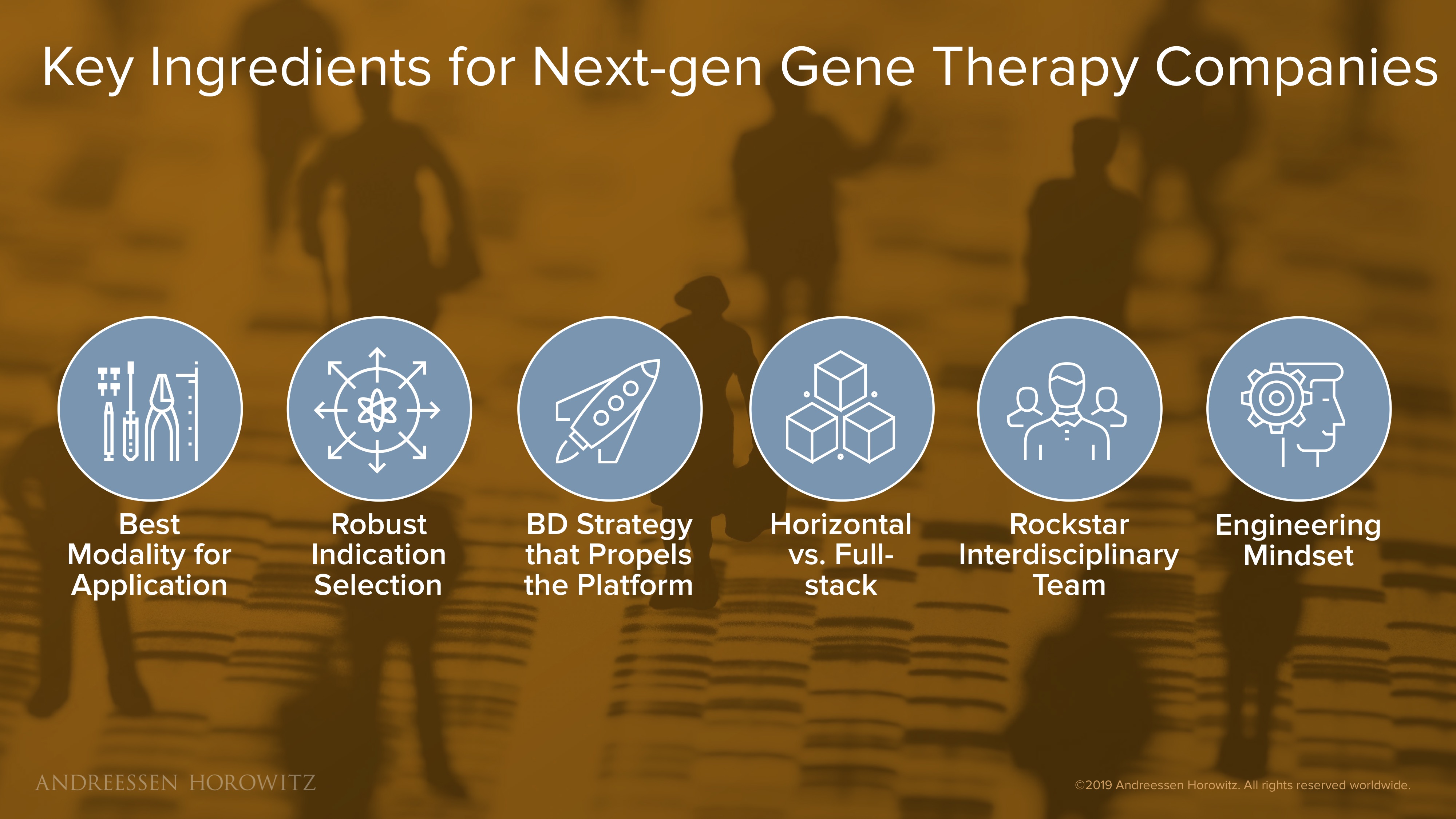
Key Ingredients for Next-gen Gene Therapy Companies
Below are some of the key components we look for in companies innovating in this space.
- Do you have the best modality for the application? There is no one-size-fits-all modality, nor one best tool for the job. The ironic problem that we have in this field is that the pace of innovation grants us a shiny new genome engineering tool every few months (or even every few weeks). But the latest, most advanced, and—by definition—least validated system is not always the best tool. There are always a variety of platforms and techniques available, and no clear winner for each application. You need the simplest technologies for your use case, without adding layers of complexity just to be novel.
- Do you have a robust indication selection? Indication selection is fundamental for all drug companies, but perhaps even more crucial for gene therapy companies because of the broad applicability of these tools. Aside from picking an application based on the understanding of your modality and an unmet clinical need, it is also important to evaluate the appropriate balance between investing in low-risk/modest-reward programs versus high-impact/high-risk. There is no single best clinical pipeline portfolio strategy, but winners will have invested ample time upfront to think it through.
- Do you have a strong BD strategy? There is ample capital out there, and many possible applications. Executing a BD strategy that propels the platform forward is super important. Take strong partnerships that are not only good deals from a financial standpoint, but also showcase unique capabilities of the platform and help craft the vision of the company.
- Do you go horizontal, or full-stack? Is the company is better served as a horizontal based platform company, or an integrated full stack company? Does the value of this technology come from diverse application and partnering broadly, or does the nature of the product make it better suited to be developed entirely in house, in one fully vertically integrated company?
- Do you have interdisciplinary teams? Because of the complex nature of these medicines, now more than ever before, having interdisciplinary teams is key. You likely will need the collaboration of molecular biologists, protein engineers, ML-natives, data scientists, clinicians, even manufacturing experts.
- Do you have an engineering mindset? An engineering mindset runs through not only development of core product or drug, but also throughout the entire organization. In the midst of a rapidly exciting and growing field (from the science, clinical, business and regulatory landscape), it is important to be nimble and iterate quickly.
Finally…. No disease will be left untreatable
As the technology, infrastructure and economics of these remarkable drugs continue to progress, it is not too far-fetched to conceive of a future in which many of our most devastating diseases can be targeted and treatable with these genome engineering tools. There has never been a more exciting time for gene therapy innovation. If you are building a company in this space or have ideas on how to tackle these key issues, our door is always open. Together, we can make this new paradigm of powerful drugs common place and accessible for all the patients who need it the most.
For exclusive access to download this complete deck, please sign up for our a16z Bio Newsletter here.
- Journal Club: Slaying the Sleeper Cells of Aging
- Evaluating AI in Bio: How to Know Whether it is Worth the Work
- Platform-Disease Fit: Why You Need It and How to Find It
- Journal Club: Turning a Toxin into a Genome Editing Tool
- Upgrading cell and gene therapy “software” for safety; Pharma picks their PROTAC horses; The first healthtech giant; and more