For generations, the field of biology has been largely defined by its discoveries. Now, because many crucial aspects of biology have begun the transition from an empirical science to an engineering discipline, the pendulum is shifting from “What can we discover?” to “What can we solve?”. Nowhere is this perhaps more evident than in the global scientific community’s collective response to combat a global pandemic—pushing us even further into one of the most prolific periods in the world of bio in both scientific and commercial development we have ever seen.
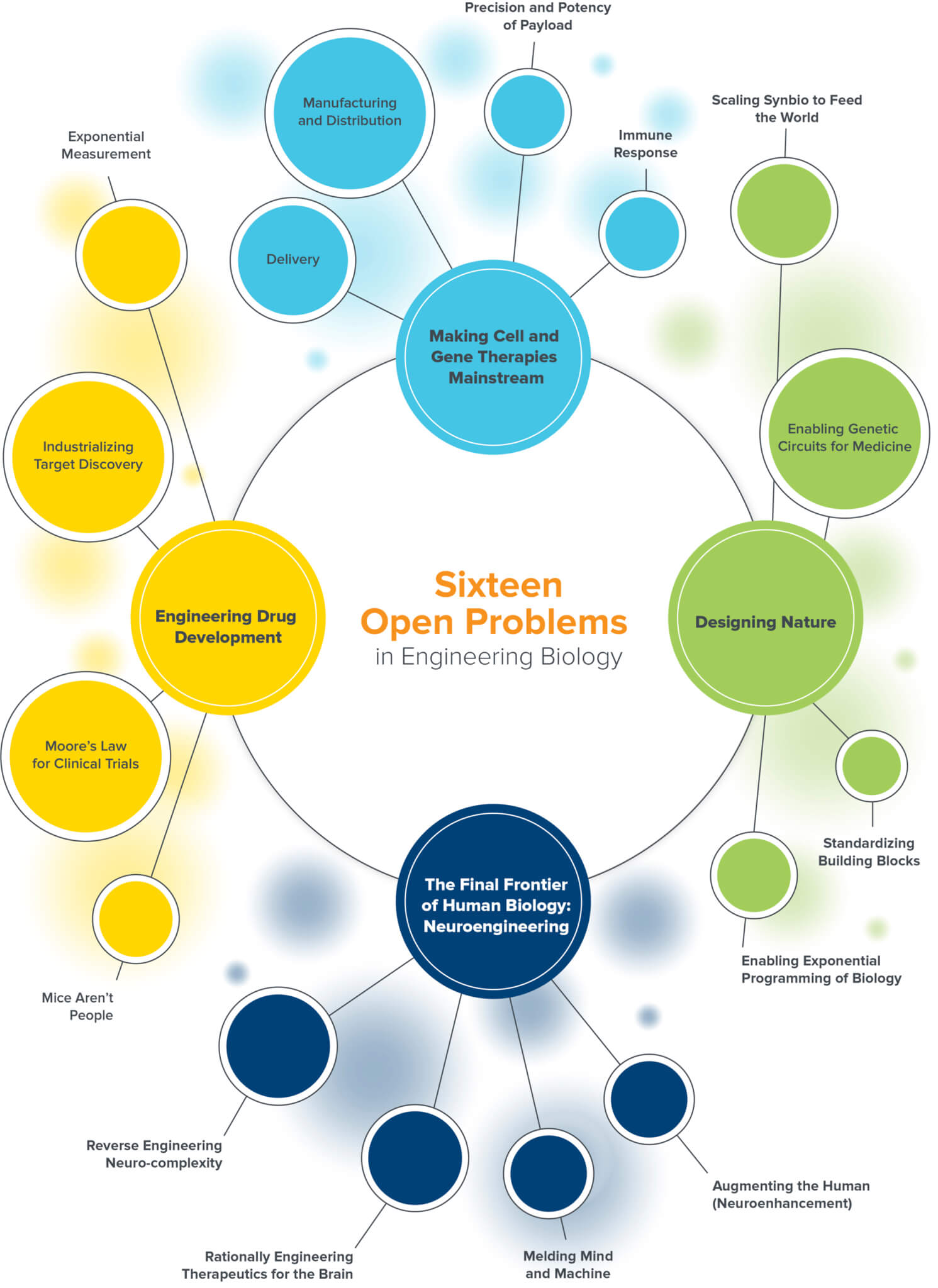
As our engineering toolkit in bio expands to be more powerful than ever before, and the infrastructure to deliver, produce, and scale these solutions comes increasingly online, a whole new world of problems in biology begin to feel approachable. Here are the 16 biggest and potentially most rewarding challenges we see coming in the world of bio.
Because many crucial aspects of biology have begun the transition from an empirical science to an engineering discipline, the pendulum is shifting from “What can we discover?” to “What can we solve?
Engineering Drug Development
Our drug development system is in desperate need of improvement. It costs an average of $2.6Bn to bring just one new drug to the public—and the medicines we develop only work 5% of the time in the human body. In fact, the cost of developing a new drug has doubled this past decade, following Eroom’s law (Moore’s Law backwards). This is in part because of the long-standing industry reliance on empirical testing and highly manual labor; add to that science risk, a complex regulatory landscape and the long, precarious road of clinical testing of a new drug, and you begin to understand why the failure rates are so high. Among many challenges, when it comes to drug discovery and development, we need to 1) reduce discovery development cost; 2) increase drug efficacy; and 3) expedite the speed of this laborious process. There are a few key ways we can begin to do this.
1) Mice Aren’t People (a.k.a., Better Models). We can cure a lot of stuff in mice; only a tiny fraction of these seemingly potent cures ever make it to humans. One big reason for that is because the model systems we use to test drugs—whether that’s in vitro, in mammals, or even in other people—is, in many cases, far from the original human condition. You and a mouse are very different; you and primate are still different; even you and another person are different. This imperfect translation plagues the drug development industry, particularly in complex diseases. We need better and more sophisticated models for drug development that can be predictive. Global challenges like COVID-19 highlight the need for accurate, predictive modeling that can greatly streamline R&D. Improvements could come from genome editing tools that allow us to create more accurate patient-derived cell cultures or better mouse models that better mimic complex human diseases. State-of-the-art AI can be used in combination with multi-dimensional data plus real world evidence to tie together these disparate data sets, uncovering insights about disease we might never otherwise have seen. New 3D cell culturing methods that take into account structure of tissues—like “organoids,” or maybe even one day model human organs entirely—could potentially mimic the physiology or microenvironment of a particular pathology. Ultimately, we will think of all these models not each as a single data point, but as an entire universe of features for modeling diseases more accurately.
2) Exponential Measurement Tools. You can only engineer what you can measure. The massive advances in genomics and exponentially plummeting costs of DNA sequencing have transformed biology into a data science. But human biology is a multidimensional universe of enmeshed signals that genomics can only begin to approximate.
The next generation of tools we need will continue unlock our ability to understand life at an increasing number of biological dimensions (proteomic, epigenomic, metabolomic, etc.); at different resolutions in from a spatial perspective (2D,3D,4D); precision (single cell, protein, atom); format (still image vs. dynamic video); and speed (higher throughput, in parallel). Adding new measurement axes to that list, even relatively imprecise ones (like: hit/not hit) can still be very powerful on a large scale. And different axes can be combined to unlock entirely new kinds of analyses (e.g., high-throughput spatial transcriptomics, real-time cryo-electron microscopy). The same way sequencing the DNA of single cells led us to a new universe of drug development, the next generation of platforms and screening techniques for additional biological dimensions will allow us to drug entirely new targets of disease.
The same way sequencing DNA led us to a new universe of drug development, the next generation of platforms and screening techniques for additional biological dimensions will allow us to drug entirely new targets of disease.
3) Moore’s Law for Clinical Trials. If clinical trials were 1/10th the cost or time, we would have cured many seemingly intractable diseases by now. The global pandemic we are now in is driving home the massive life-and-death importance of transforming these old paradigms to immensely increasing the speed to get new diagnostics, treatments, and vaccines. And these trials are only becoming much more costly as our therapeutic modalities become ever more complex. Even when the biology of a disease is straightforward and well known, the economics of market + cost of trials often stops the biopharma industry from developing a drug that might otherwise save millions of patients.
There are many entry points for tech to help reengineer the clinical trial system to be faster, more efficient, and more impactful with less: software that can automate patient recruitment and trial set up; better infrastructure for collecting patient data; synthetic control arms that use real world evidence. The new realities catalyzed by COVID-19 have spurred the shift to telemedicine and other at-home techniques. New ML techniques and deep longitudinal biomedical data are also increasing our ability to predict the probability of success of a drug’s clinical trial over a broad range of indications—expediting the trial process by allowing biotechs to focus on assets that are most likely to succeed. While the ultimate goal is to reverse Eroom’s law, even a modest increase in predictive accuracy that can help prioritization could have massive savings for the industry.
Just a 5-10% efficiency on cost or time—a Moore’s Law for clinical trials—would mean that in 7 years, it might cost half as much to run a new therapeutic through clinical trials, and an order of magnitude more diseases cured.
4) Industrializing Discovery. The way most disease biology is discovered starts with, well, sick people. In other words, we start with the disease phenotype and try to understand what’s going on in the biology that creates these conditions. Then, we may try to empirically throw compounds at disease models until something sticks or painstakingly attempt to elucidate a specific pathway. The advent of modern, state-of-the-art AI/ML technologies combined with our increasing wealth of biomedical data are rapidly making these classical methods obsolete.
From computational tools that analyze structure-activity relationships to applying AI/ML on cell images for high-throughput phenotypic screens, or sifting through multi-omic signatures, we are just at the cusp of what is possible. The next wave of AI/ML tools we need are platforms that can learn entirely new and unique features (ones that scientists or physicians never would have thought to have correlated with a specific pathophysiology), harness and utilize small datasets (extremely valuable for indications where data is sparse or low quality) or combine insights from disparate disciplines. Add to that mix new hardware for robotic data generation that can generate high-throughput, fresh datasets tailored for AI/ML and we may one day be able to scale research and development that used to take years (or was previously impossible) in a matter of days.
Making Cell and Gene Therapies Mainstream
Engineering cells and genes in order to treat disease has already given us some incredible medical breakthroughs: immune cells trained to seek and kill cancer; gene therapies that cure some devastating genetic diseases. But there are still many engineering challenges to solve before these medicines work for more diseases, safely and effectively, and at scale—in order to get these treatments to the patients that need them the most.
5) Sculpting Immune Response. The immunogenicity of gene therapies—ie, their ability to provoke an unwanted immune system reaction—has always been one of the biggest challenges for the gene therapy industry, with some high profile clinical debacles in the past and present as harrowing reminders. Immune system responses can be caused by many different aspects of the therapy, from the delivery vector itself to the “cargo” inside the vector. We need tools to help us sculpt an immune response for maximum potency but minimum toxicity: platforms to help screen for immunogenicity, plus new capsids or delivery systems that minimize it. For cell therapies, we need new control mechanisms that utilize synthetic biology to make these therapies safer: “kill switches”; second generation CAR Ts that default to “off”; or programming cells to make them less “visible” to the immune system. Engineering new promoters and regulatory elements for cell/environment-specificity and toggleable control in general can be key for all cell and gene therapies. More immediately, better diagnostic systems could also help us avoid the problem altogether, making sure we know if a patient has an immunogenic predisposition early on.
6) Engineering Delivery Systems. One of the most crucial technical components of gene therapy is the delivery system. Delivering these medicines to a specific target organ or even cell type is no trivial feat. Right now, many of our current delivery systems are plagued with low tissue level precision, low efficiency, and high immunogenicity (causing immune reactions). For ex-vivo therapies engineered outside of the body (e.g., cancer immunotherapies, sickle cell, beta thalassemia, etc.), we need delivery mechanisms that can disrupt the cell membrane while minimizing damage to the cellular membrane and core intracellular proteins. There is an explosion of tools coming that can be harnessed to solve a huge range of these delivery challenges, from viral and non-viral in the biological spectrum, to chemical polymers, physical hardware, or even utilizing cells themselves to carry cargo. In short, in each of these different categories we need reliable platforms that are modular, can be iteratively engineered, and easily tailored for a breadth of patients.
7) Perfecting the Payload. Delivery is one thing; what exactly is being delivered is another. Off-target effects, low efficiency, and side effects from immunogenicity of the protein itself (ie, the “payload”) are also critical problems for the field to solve. For gene therapies, even a tiny unintended off-target effect or “genetic scar” can trigger unwanted mutations or new cancers. We need to engineer new tools with greater control and precision: again, more on/off switches; more control mechanisms, such as photocleavable protein domains; editing tools that don’t require double stranded breaking mechanisms; endogenous systems that might avoid CRISPR altogether. There are a lot of new enzymes that are being discovered that give us the ability to edit new dimensions of the genome safely and precisely. For cell therapies, we need more new technologies that will enhance the functionality and potency of T cells. Engineering new chimeric antigen receptor (CAR T) designs and systems will fuel much of the next generation of immunotherapies. From armoured CAR Ts to self-driving CAR Ts to self-destructing CAR Ts, we need new CAR T modalities to enhance trafficking, avoid tumor suppression and escape, and ultimately boost potency of the immune response.
8) Manufacturing and Distribution. There is a large, hairy system and deep supply chain that has to work smoothly behind the scenes in order to make and deliver these extremely complex drugs to patients. As many of these novel gene therapies are made for each individual patient, the current systems are seeing huge manufacturing bottlenecks and long contract research organization (CRO) wait times, sometimes even up to a year. We need to apply the tools of automation and robotics to scale the manufacturing steps of these therapies, from redesigning the logistical processes to using software to intelligently manage critical parts of the supply chain.
We may also be able to leapfrog some of these logistics by developing better allogeneic cells, or universal, “off-the-shelf” donor cells, that can help to democratize cellular immunotherapies. New approaches that can produce allogeneic T-cells with minimal potential for life-threatening graft-versus-host disease or new cell types such as NK-cells could also be major breakthroughs. The true disruptor, though, might one day be avoiding any type of cellular production altogether—perhaps by methods like delivering nanoparticles into the cells to directly induce CAR expression in vivo—sidestepping the entire need for this herculean manufacturing and logistics infrastructure.
Designing Nature
We are finally entering an era in which the engineering principles of abstraction, modularity, and hierarchy can be applied towards the design of biology itself—i.e., the field of synthetic biology. This means dreams of engineering organisms programmed to sense and combat disease; living materials that can self-repair; or highly efficient biological systems that can produce energy, are beginning to seem closer to reality than science fiction. There is still an enormous amount of infrastructure to be built around this space to make applications like these a reality.
We are finally entering an era in which the engineering principles of abstraction, modularity, and hierarchy can be applied towards the design of biology itself.
9) Better Building Blocks. Over the past two decades, we’ve been able to engineer increasingly complex genetic circuits to perform more and more sophisticated cellular functions. Yet programming genetic circuits often still requires many more design->build->test->cycles than the highly predictable, efficient cycles of other traditional engineering disciplines. Biology is incredibly complex; our only way to manage this is to break it down into standardized biological modules—Lego-like building blocks—that will allow us to more easily build and swap core components in a complex genetic circuit, in a predictable manner. In addition to these interchangeable Lego blocks, the field will also need many new tools to both understand and ultimately design nature’s complex biophysical systems. New software platforms that can better simulate, predict and even prototype biological circuitry interaction will be crucial innovations driving us into the next era of truly designing biology.
10) Programming Biology… at Scale. Though we can read (sequence DNA) and write (synthesize DNA) quickly, our ability to actually execute (make large numbers of genetic modifications) or program biology in mammalian cells is still in its nascent stages. New molecular tools such as CRISPR allow us to make precisely targeted edits, but the reality is it takes months to do just a handful of edits. In computer science speak, a bio I/O system is still very slow. To truly accelerate the future of synthetic biology, we need tools to be able to do thousands of edits in mammalian cells at once—which would require interdisciplinary innovations in biology, chemistry, hardware, and automation. We have seen some of these innovations already in simpler microbes, like bacteria or yeast, but there are a number of hurdles to cross before we see these tools and platforms in the realm of complex mammalian cells: human cells take much longer to divide than bacteria; off-target effects are more lethal; it takes longer for things to be absorbed into a human cell. These are big, audacious challenges for the field, but solving them would mean we could literally engineer cells from scratch, rapidly creating and democratizing new cellular machines for every aspect of life and industry.
11) Genetic Circuits for Medicine. One of synthetic biology’s greatest promises is the potential to program “living cellular machines” as therapeutics: engineered cells that can detect multiple disease signatures, trigger sophisticated therapeutic mechanisms, and turn off after sensing the removal of a disease state. These cellular machines could be the basis of powerful new systems for us to detect, diagnose, and one day even treat disease. So far, visions of these sophisticated “theranostic” (therapeutic + diagnostic) biological machines (which would require complex engineering features like multi-input logic gates, precise transcriptional control and other modules for sense, memory, and biocomputation) are more proof-of-concept than reality. But some of this functionality already does exist—it’s incorporating it into a sophisticated whole cell “machine” that’s so hard. So one way to make headway would be to incorporate those components into beefing up existing therapeutics. We are already seeing this in the form of synthetic Notch (synNotch) receptors; universal and programmable (SUPRA) CARs; even pancreatic cells that sense and release hormones in response to food consumption. The next generation of winners in this space will be those that can harness increasingly complex circuitry one module at a time.
12) Synbio for the World. Though petroleum based processes have created the modern world around us, they’ve also, well, created the modern world around us—with all its ecological crises. Biology itself may be our best manufacturing system. Using genetically engineered biological systems, we have been able to produce biomaterials and biomolecules for use in medicines, food, beverage, textiles and industrial applications. And not just simple molecules, but leathers, textiles, even meat. But the problem holding back this entire space is the ability to efficiently scale biomanufacturing. The current production costs for many of these products are exorbitant, making the unit economics of these products impractical for widespread usage (this of course was part of the biofuels debacle). For this kind of manufacturing to reach true scale, we need to be able to use host organisms that can produce with higher yield and minimal undesirable by-products. Scalable platforms that can efficiently reconstitute and mimic the natural environment of the 3D scaffold (like for meats, leathers, silk, etc.) will also be essential. And then we need new advances in software and hardware to automate many crucial elements of the industrial scaling process: fermentation, tissue engineering, even better analytics so we know how to refine and iterate our manufacturing processes further.
Neuroengineering
The central nervous system (CNS)—one of the most important and complex systems in human biology—has arguably been medicine’s most enigmatic field. Pain, depression, Alzheimer’s, spinal cord injury, and countless other incurable diseases and conditions point to our desperate need for solutions in this area. But recent innovations in both software and hardware are opening the door to engineering the neurological systems at an unprecedented resolution. And beyond treating disease, the next generation of hardware in brain-computer interfaces might even one day transform the way we exercise, sleep, entertain ourselves, or interact with the world.
13) Reverse-Engineering Neuro-Complexity. The number of connections in the brain dwarf the number of observable galaxies in the universe. When it comes to neurological diseases and mental health disorders—complex conditions where we understand relatively little about the underlying biology—we desperately need much higher resolution models to give us actionable insights. This begins with simply trying to see the full brain and CNS, with “wiring diagrams” that map the entire brain’s neuronal connections, synapse by synapse; or tools or software that can scan and image the entire brain, combined with software or ML algorithms that can then rapidly accelerate tracing these neuronal connections. We can also “let nature do the work” by bioengineering brain models from human tissue to illuminate what we don’t understand: brain organoids, which are simplified 3D recapitulation of the core components of the brain, are one example of this. But we need to increase the anatomical accuracy of those organoids, as well as develop better ways to model the entire circuit of neuro-architecture. Making the fabrication and construction of these models a more streamlined repeatable procedure will also help make this tool much more useful and more widely adopted.
14) Rationally Engineering Therapeutics for the Brain. The development of CNS drugs has been met with herculean setbacks. With significantly higher clinical failure rates (as high as 100% in some diseases like Alzheimer’s), CNS drugs also face longer development and regulatory review times. And even when successful, because of the breadth and complexity of each CNS disease, there will never be one silver bullet drug. Treating these diseases will likely come from a broad armament of tools that will span from the molecular level (cell and gene therapies that target a root cause; advances in stem cells and regenerative medicine) to reengineering our behaviors through digital means. Mobile apps have already ushered in a new era of digital therapeutics for mental health issues including depression, insomnia, or stress. Ever improving higher resolution VR technologies that increase the degree of realism unprecedented in immersion based treatments may also provide us with new powerful and effective treatments for disorders like depression and PTSD.
15) Melding Mind and Machine. Though science fiction ideas of interacting with our environment or connecting to computers (or AI) with only our thoughts have long since captured the fascination of humankind, there are still many steps to cross before brain signals can be harnessed and translated into intentions and actions. We are not there yet, but brain-computer-interfaces (BCIs)—devices that connect the brain to external hardware—have shown spectacular proof-of-concept demonstrations with BCI-controlled robotic limbs that rejuvenate movement for completely paralyzed patients, brain implants that restore vision in the blind, or electrical stimulation neurotechnologies that enable the spinal cord injured to walk. What started off as medical devices for analyzing electroencephalogram brain signals have blossomed into a bleeding edge field that promises to not only improve the lives of the disabled, but also completely transform our paradigm of interaction with the world.
Translation of these early concepts to much higher fidelity, reliable and precise clinical products is no trivial feat—in part because of the enormous amount of electrical information and the imprecise correlation with psychological phenomena. Any kind of BCI will need to take into account how invasive (or non-invasive) the end-product is and the inherent tradeoffs. Non-invasive BCI approaches are safer and easier to manage, but their efficacy is often hindered by our brain’s rigid shield, the skull (which impacts the fidelity and accuracy of complex signal processing). Development of AI and ML algorithms that can analyze and decode this neural activity (while mitigating invasiveness) might turbocharge the field. And the hardware challenges are no mean feat either: robust electronics must use safe, biocompatible, non-immunogenic materials, and be cost efficient to manufacture.
16) Augmenting the Human. How far away are we from technologies that will push beyond the fundamental baselines of human cognition and function? That will improve memory, boost IQ /EQ, maybe even give us new motor abilities? With an engineering approach, we can begin to target these areas as well—going beyond the concept of development with a purely disease-based mindset, and towards improving function. Could a therapy for Alzheimer’s Disease improve memory for people without the disease—perhaps through editing and regenerating neurons, or manipulate hormonal or pharmacological mechanisms that allow us to store or edit memories? On the hardware side, we are already seeing early glimpses of electronics that take advantage of neurological signals to modulate our brain activity to reduce stress, enhance sleep, or even boost efficiency of physical training. Whether through new tools like these “electroceuticals” or through small molecule, protein, or cellular therapies, functional improvement outside of disease will become a natural next frontier.
These 16 problems are, of course, just the beginning. These themes serve to highlight the degree of the pervasiveness of engineering’s impact. We no longer have to make the argument that engineering biology is a thing. The real question is what becomes possible when engineered biology has pervaded not just these problems—but everything.