Intro
We have long believed that AI will fundamentally reshape biotech and healthcare, positioning us at the brink of an AI-driven Industrial Revolution. But when will we see this payoff? Put more boldly, when will the majority of new drugs be designed with AI? The first wave of therapeutic candidates designed and optimized by AI/ML tools are making their way through clinical trials now, and new NDA/BLA applications referencing AI are accelerating rapidly. However, it’s crucial to recognize that solely counting the number of approved AI-enabled medicines is a lagging indicator of success, overlooking the expanse of treatments still in development (and the long time lag due to clinical trials).
To contextualize this point, consider the evolution of the natural language processing (NLP) field. Initially, GPT-2 marked a significant step forward but had notable limitations in areas like fact-checking and contextual understanding. In contrast, GPT-4 and other large language models (LLMs) have now catalyzed a generative AI renaissance, anticipated to contribute trillions to the global economy. In biotech and health, we are in the early innings (the equivalent of the GPT-2 era) of a significant shift in life sciences R&D driven by AI, and view the continued acceleration of AI research, the increasing number of AI-driven pharma partnerships, and the tangible time- and cost-savings in drug development realized by AI-enabled discovery platforms to be material leading indicators flagging an imminent and substantial transformation in the life sciences ecosystem.
How does this future get realized? We recently wrote about the “Jobs to be Done” (JTBD) for AI in enterprise healthcare. In life sciences, the combined existence of large amounts of complex, multimodal data paired with labor-intensive, high cost tasks creates an optimal opportunity for AI to fundamentally change the future of an entire field. Here, we describe our view of the JTBD in life sciences where we believe AI will have the largest impact.
Andreessen Horowitz analysis
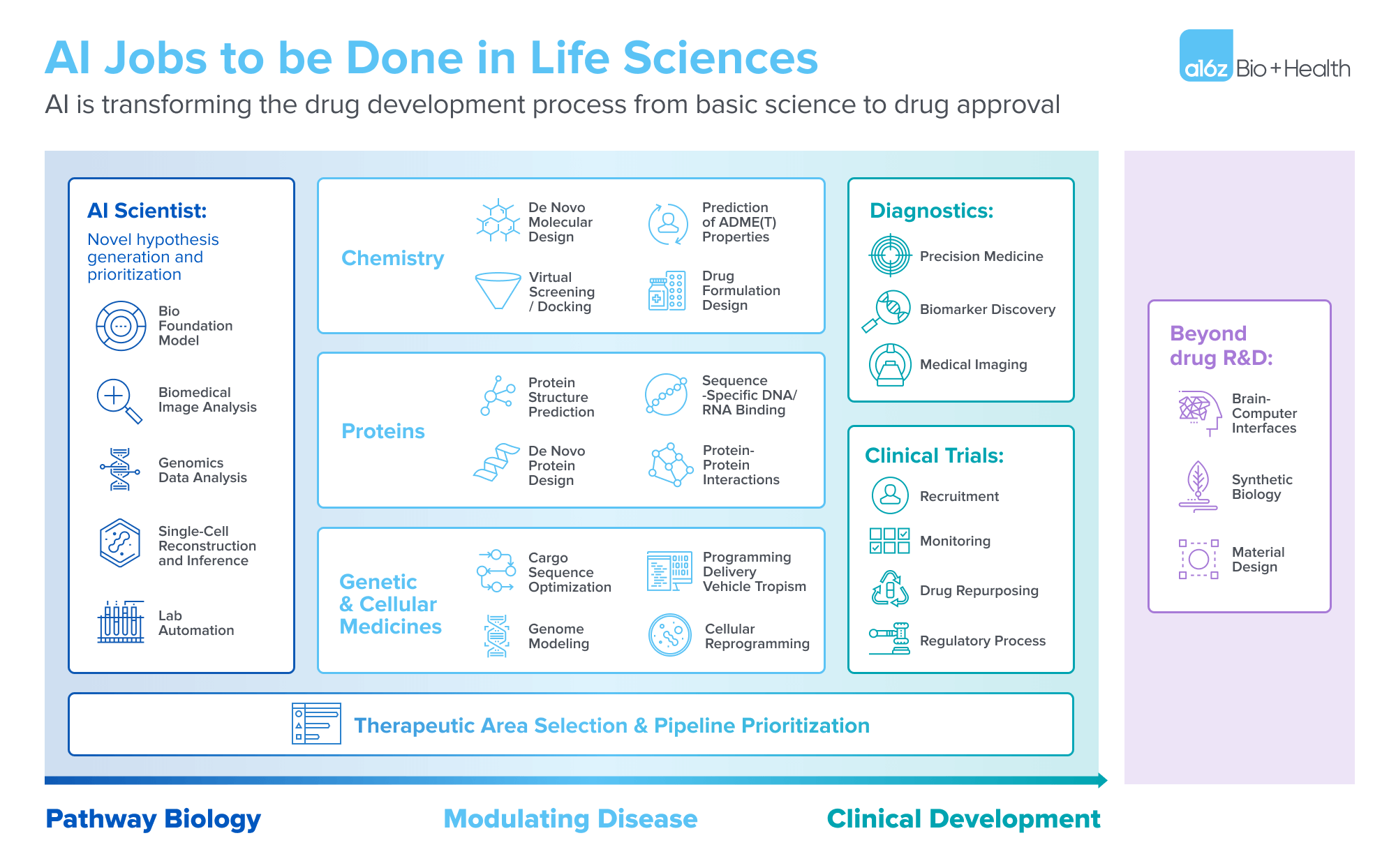
AI jobs to be done in life sciences
Human pathway biology
Hypothesis generation and prioritization lies at the heart of scientific discovery in life sciences. It’s a labor-intensive process that demands sifting through extensive research literature to identify promising avenues for investigation. Here, the AI Scientist emerges as a game-changer, automating literature reviews, analysis of experimental data, and hypothesis generation, with potential to achieve superhuman capabilities. State-of-the-art LLMs can assimilate learnings from the entire corpus of scientific work produced throughout human history, a scale unattainable by any single human.
While we’re only starting to see early signs of the immense promise of such an approach (e.g. insitro, Future House, SciSpace, BioAge), it’s easy to envision a near future where researchers frequently consult an AI Scientist for pressure-testing new ideas and helping prepare research proposals. This efficiency extends to experimental design and data analysis, where AI can unveil hidden insights from complex datasets, which can in turn inform new hypotheses. Moreover, digital twin technology, spanning from whole-cell to whole-human simulations, foretells a future of in-silico testing and systemic understanding of diseases. Coupled with AI-driven lab automation, there’s potential to minimize human intervention, thereby establishing a continuous learning loop that accelerates the cycle of discovery.
AI for TA selection/pipeline prioritization
One of the most important decisions a therapeutic platform company must make (and continually re-make) is what overall therapeutic areas and specific therapeutic targets to point their technology towards. This is such a crucial task that an entire sector of consulting companies has been built around it, servicing both small biotech and large biopharma. Successful therapeutic area selection and pipeline prioritization requires the synthesis of large amounts of both historical data and the ability to accurately predict future competitive landscapes in order to inform strategic decisions for a company’s pipeline. We view this area as a compelling job for AI, where large amounts of complex data must be synthesized and the ultimate result is relatively forgiving to mistakes.
AI for preclinical development
AI is set to disrupt virtually every aspect of the preclinical drug development lifecycle, from target discovery to final formulation. In fact, across both biotech and pharma, we’re already witnessing tangible reductions in therapeutic discovery & development timelines in the informed design of efficacious small molecules, proteins, or cell and gene therapies.
We believe the era of serendipitous biologics discovery will soon be replaced by advances in diffusion and protein language models combined with previous AI technologies for classification, resulting in fully conditional generation of proteins with defined function, or even new-to-nature mechanisms. Together, these models could enable precise targeting of distinct binding epitopes, tunable enzymatic processes, or even conditional protein activity that will no doubt further our ability to develop innovative therapies. The future of biologics discovery lies in leveraging diffusion and protein language models to gain an atomistic understanding of the protein “interactome” and to enhance AI-accelerated dynamics simulations, enhancing our ability to develop innovative therapies and deepen our understanding of disease mechanisms.
Beyond protein design, recent advances in reference-free protein-ligand structure prediction and generative chemistry approaches (e.g. Genesis Therapeutics) are not only revealing novel binding sites for structurally-unresolved proteins, but also providing methods to elaborate specific, developable, and potent chemical matter for them. Additionally, the continued development of tools to de-risk the optimization of compelling hits (e.g. Inductive Bio) suggests we’re on the precipice of a new medicinal chemistry paradigm.
Downstream of discovery, formulation and delivery mechanisms are arguably as crucial as the drug molecule itself, as these dictate exposure and drug-target interactions. Rather than relying on a bespoke, trial-and-error approach for discovering optimal therapeutic formulations, the use of algorithms to predict drug release and interactions with excipients is shifting this final stage of development from an artisanal to a more systematic process (e.g. Mana Bio, Dyno Therapeutics).
However, making these therapeutic candidates is only half of the battle, and we should also look to ways where AI can improve the probability that promising biomedical research can traverse the so-called “valley of [developmental] death” to successfully reach clinical trials and ultimately market approval. One such avenue involves incorporating virtual animal models that can pinpoint compounds with better translational pharmacokinetics or those with potential toxicities, ultimately requiring fewer real-world animal studies.
Saving time and dollars on the clinical side
Over half of the total investment in the drug development process is spent during the clinical development phase. We view the seamless integration of AI into jobs across clinical development as one of the clearest paths to reducing the cost and timelines for drug development, and ultimately improving patient care. Clinical development yields a vast amount of varied data, offering ample opportunities for AI-driven solutions to refine multiple aspects of clinical development. Even modest improvements could significantly reduce both the high costs and extended timelines of drug development, a compelling value proposition to pharma. For example, LLM guided clinical trial design and protocol drafting could level the playing field between small biotechs and large pharma. Additionally, AI-driven patient selection for clinical trials could enroll patients more effectively and precisely, improving recruitment across sites and overall trial success rates.
Given the vast amounts of complex, real world data being increasingly collected during clinical development, AI-powered data analysis could identify both signals of efficacy and safety in real time, distinguishing between treatment correlation and causation, and ultimately improving the quality of drug approvals. And finally, instead of more regulation for AI—we need AI for the regulatory process! AI enabled tools could be used to streamline the IND application and review process, reducing cost on both sides and increasing the rate of successfully submitted drug approvals.
Beyond drug R&D
AI is also poised to play a crucial role in the bioeconomy more broadly.
Biomanufacturing is fundamentally anchored on enzymatic processes, which have proven difficult to optimize for the rigors of manufacturing settings like bioreactors. Introducing AI-based protein design capabilities could enable the generation of new-to-nature enzymes fine-tuned for specific manufacturing tasks. This could potentially unlock unit economics that could rival those of traditional manufacturing practices.
AI stands as a formidable ally in the battle against climate change, and we’re already seeing examples of transformer-based AI models being employed to design novel enzymes and materials optimized for more efficient carbon capture.
Lastly, the field of brain-computer interfaces is set to gain significantly from AI advances, enabling both innovative non-invasive approaches like silent speech recognition devices, and enhanced decoding performance for emerging implantable interfaces, thereby paving the way for the restoration of lost neural functions.
Conclusion
AI has emerged as a powerful tool capable of interpreting the complex language of biology, and as per our life science AI thesis, we are betting on its ability to tackle our greatest challenges across life sciences. We firmly believe that over time, AI will infiltrate the entire spectrum of “Jobs to be Done” across drug discovery and development—from hypothesis generation to clinical trials, and beyond. As we continue to witness AI’s transformative impact, we invite you to keep an eye out for additional in-depth explorations into the Jobs that excite us the most.
-

Becky Pferdehirt is an investing partner on the Bio + Health team, focusing on early stage companies building technology platforms for therapeutic discovery and development.
-

Bryan Faust is an investing partner on the Bio + Health team, focused on life science investment opportunities.
-

Zak Doric is an investing partner on the Bio + Health team, focused on biotechnology companies.
-

Vijay Pande is a former GP and founder of the Bio + Health team at Andreessen Horowitz, focused on the cross-section of biology and computer science.

